Building Patient Trust with Next-Generation Clinical Trial Training
Patient trust is critical to ensuring adequate enrollment and retention of clinical trial participants. If patients lack trust in the capabilities of the investigators, nurses and other staff conducting a clinical trial, they are less likely to enroll in a study, and even if they do, they are more likely to drop out if their trust is rocked at any point by shaky performance of trial procedures.
The way that clinical researchers are trained can play a key role in building the necessary trust. The more confidence patients have in the knowledge and abilities of research staff, the more comfortable they are likely to be with the trial processes—and the more likely they are to enroll and remain in the trial for the duration.
Simulation-based training, such as that provided by Pro-ficiency, can play a crucial role in increasing patient comfort levels with clinical trial participation. By offering realistic scenarios, this type of training lets clinical research professionals hone their skills in a risk-free environment. This immersive approach based on realistic scenarios bolsters their qualification and competence, so researchers are better equipped to conduct trial procedures smoothly and effectively, leading to a more comfortable experience for patients.
Most trial participants are unaware of how sponsors conduct training on protocols or that the traditional approach relies on slide decks, a method that most patients would likely view as worrying. Additionally, the pharma industry in general faces challenges related to mistrust. In clinical research, any uncertainty displayed in describing or performing protocol procedures can exacerbate any reservations a potential study participant may feel. And this can drive both low enrollment and participant drop-out after a study begins.
Patients want to be confident that the investigator or research nurse is providing the right dose on the right day, for instance, and that the team has all the tools necessary to execute the trial correctly. That puts a burden on research teams to demonstrate to patients that they have sufficient knowledge, skill and understanding to overcome that underlying suspicion—a burden that is difficult to overcome without thorough training.
A 2021 blog post from Medidata emphasizes the foundation of trust in clinical trials. It highlights the needs for clear communication to ensure that participants comprehend study procedures early in the process. This then sets the stage for more impactful communications throughout the trial, which further fosters trust between the patient and the research team.
Medidata writes, “To build trust with clinical trial participants, it’s important they understand precisely what is being done and why, early in the process. With that established, the communications that follow will have more impact and help establish trust throughout the trial experience.“
And sometimes, a simple solution is the most effective. In this case, innovative training that successfully alters the behavior of healthcare providers conducting clinical trials and improves performance of all clinical trial procedures can play a key role in building and retaining patient confidence and trust.
Training that ensures competence in all trial procedures can play a major role in presenting trial information in a way that assures patients and their families that participation will be a positive experience.
Proven Performance Results
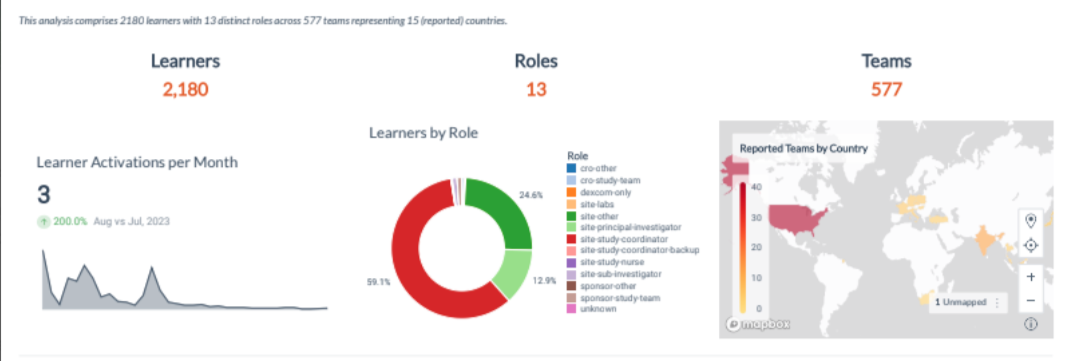
Simulation training, such as that provided by Pro-ficiency, provides research teams with the chance to practice. Immediate feedback helps to guide research staff to develop better decision-making skills. The intuitive dashboard allows tracking of performance within and across sites.
This training approach immerses coordinators, investigators and other research staff in realistic scenarios, which helps to ensure that they are well-prepared to both describe and perform procedures accurately, giving participants more confidence in a trial. By providing a risk-free environment to practice and gain skill and understanding, a simulation-based training platform enables research staff to confidently interact with patients, conveying accurate information and addressing any concerns they may have. This ensures that patients receive clear and consistent communication throughout their participation in the trial, enhancing their overall experience and likelihood of enrollment.
Real-time performance analytics of sites allows sponsors to see where gaps or weak areas may exist and to focus on the areas where training is most needed.
This type of training has proven over time to be extremely effective in improving performance. For instance, Elio Mazzone et al. noted in a paper in the August 2021 Annals of Surgery that proficiency-based progression simulation training was more effective than traditional training in reducing procedural errors and increasing the number of correct steps taken. A key, the paper said, lies in continuous feedback and the ability to repeat tasks until they are done correctly.
Why? Virtual simulations of realistic, protocol-specific scenarios provide the opportunity to replicate real-world situations, providing an environment that invites engagement and builds accessible professional knowledge, rather than providing just abstract learning of content from static materials. At its heart, simulation technology achieves a complex, meaningful interactivity, allowing users to construct and test hypotheses and receive feedback in response to their actions in a risk-free, persistent environment. Learners can actively and rigorously develop needed skills by facing simulated challenges, seen in David Hadden’s previous protocol deviation article.
And the proof is in the pudding. In 2010, an analysis was conducted of data from a country-wide deployment in East Africa for the CDC. Every healthcare professional in the country was trained on a rigorous HIV treatment protocol
One past webinar, Imagine a world without Protocol Deviations’, illustrated the results of that training. A green circle represents a clinician who executed a simulated patient encounter with an acceptable level of clinical variance. The graphic on the left shows a pass rate of 69%. The graphic on the right shows that the pass rate increased to 97% after simulation training.
n=4026
These factors become especially important for larger, international studies. With more research sites involved, there can be greater variation among capabilities, which can lead to protocol deviations. And when international sites are involved, complications related to language and culture can challenge research sponsors to provide training that meets all the different needs at myriad sites.
Pro-ficiency provides a dashboard that displays the predictive performance analytics in real-time and spread geographically according to the distribution of sites and teams. Pro-ficiency’s dashboard of training metrics can provide just such an outline, making it easy to highlight where language or cultural barriers may exist and allowing agile development of custom modules to address those challenges. This proactive methodology has been discussed here previously.
Another benefit of simulation is that the knowledge gained tends to stick with learners better than lecture-based methods. The ability to practice tasks and procedures virtually in a risk-free environment allows employees to develop accurate muscle memory that helps them perform optimally in real-world situations.
This is especially important when it comes to developing skill and proficiency in procedures that differ from the standard of care. The ability to smoothly perform and explain novel techniques or procedures to patients is critical to ensuring patients are comfortable with trial participation.
Pro-ficiency’s platform, for instance, reduces the likelihood of errors or complications during a trial that involves novel treatments by immersing learners in lifelike scenarios. Knowing that the healthcare providers treating them during a clinical trial are well-qualified and competent in protocol-specific procedures goes a long way toward instilling trust and reassurance in patients.
Proactive problem-solving
Simulation training also allows for proactive addressing of problems in a consequence-free environment before a trial even begins. When problems are only addressed after they occur in a live clinical trial, patient trust can erode. Identifying and addressing issues up front, on the other hand, can boost patient trust in the trial and the team implementing it.
A lot of distrust in the pharma industry and in the clinical trials it sponsors can be linked to the industry’s tendency to address problems only after they occur.
But Pro-ficiency’s training packages, for instance, include analytics that are just as important as the training itself. This information can help managers track not only progress in completing the protocol-specific training, but also provide invaluable information about how well individuals and sites perform as they move through the training.
The immediate feedback and analytics offer extra support and guidance to individuals or sites struggling with any aspect of the protocol. This real-time assessment allows for timely intervention and remediation, ensuring that research staff are adequately prepared to conduct the trial procedures and interact with patients effectively. By addressing areas of difficulty promptly, Pro-ficiency contributes to the overall success of the trial and improves patient satisfaction and retention.
In other words, if staff can complete the simulated activities successfully, the training itself proves their competency. For instance, if 70% of a cohort failed in the first attempt at a simulated procedure, the organization would know that was a problem area in the protocol that needed to be addressed.
The design of the training modules allows high achievers to move easily through the material at which they already excel, while learning new procedures or practices at a suitable rate. Meanwhile, those with less knowledge or experience are carefully guided to making the correct decisions.
Investigator meetings can then be used to address any remaining pain points, using the performance analytics to guide those conversations. This will help ensure that training remains focused on crucial issues without wasting investigators’ time.
This level of training helps to ensure that investigators, coordinators, research nurses and other staff thoroughly understand the study and its procedures before they speak with patients. Not only does this help them execute the study without error, it also gives them the in-depth understanding necessary to confidently explain a trial to potential enrollees and to answer their questions easily and correctly.
With this deep understanding, research teams can focus on building relationships with patients, rather than on remembering details of the clinical trial protocol.
In conclusion, the pivotal question emerges: If patients were aware of how their HCPs were trained for trials, would they still enroll? Transparency about site performance, completion reports, and the success of top sites could potentially influence patient enrollment decisions. These considerations prompt a reevaluation of our current practices, urging us to embrace transformative approaches to clinical trial training for the benefit of all stakeholders.
Simulation-based training has a well-documented track record of producing competence among healthcare professionals. Pro-ficiency’s platform leverages this proven effectiveness, providing research staff with the skills and confidence necessary to conduct clinical trials successfully. By offering realistic simulations and comprehensive training modules, Pro-ficiency equips research staff with the tools they need to navigate protocol procedures and patient interactions with proficiency and professionalism.

Will Ewart serves as Director of Strategic Partnerships at Pro-ficiency, a leading provider of simulation-based training for clinical trials, Will plays a pivotal role in forging strong relationships with biotechnology, pharmaceutical, and contract research organizations. His primary objective is to design tailored solutions that not only mitigate protocol deviations but also fortify patient safety, ultimately elevating the overall quality of study outcomes.